Lifecore Biomedical - Post Restructuring, Inflecting Financials Over Next 3 Years, Possible Near-term Transaction
Lifecore Biomedical (LFCR)
Executive summary
Lifecore Biomedical (LFCR) – $6.65/share, $250M market cap – appears to finally be emerging from a five-year restructuring process that probably proved a little rockier than originally anticipated. The company has had significant ownership interest from activist investors since at least 2020, when investors began pushing for the company to divest its low-margin foods segment and focus exclusively on its pharmaceutical drug development business (known as a “CDMO” or contract development and manufacturing organization).
Since then, the company has successfully divested its foods businesses while expanding its CDMO business but ran into difficulties along the way. COVID hit shortly after the company announced strategic alternatives for its foods segment in Feb 2020, putting a damper on the process. Over the next few years, the company required additional capital on multiple occasions, negotiated multiple forbearance agreements and amendments with lenders, went “dark” on in its financial reporting for an extended period, almost lost its NASDAQ listing, had to restate financials, and overhauled its management and board.
Fast forward to today, the company is now a pure play CDMO with new management, and these challenges appear to be behind it. The new management team has laid out a compelling business plan that presents an interesting investment case.
My view on the situation is the following:
LFCR’s business plan looks achievable. The company finally looks like it has all the right pieces in place to execute between (1) a new management team, (2) a shored-up balance sheet and ample liquidity, (3) expanded manufacturing capacity, and (4) favorable industry dynamics. As the company fills its new capacity, operating leverage should kick in and drive a meaningful inflection in the financials of the business over the next 3 years.
There is a decent chance that LFCR gets acquired in the near-term, and the transaction could value LFCR at upwards of 2x the current stock price. Following LFCR’s restructuring and recent M&A activity, LFCR is now the only standalone public CDMO in the US. This is an industry that has seen a lot of activity from both private equity and strategic buyers in recent years, and LFCR is unique in being the last public company in the space. For what it’s worth, the company currently estimates an 80% probability of a “change in control” by May 2029 (the maturity on its term loan) and recently increased the chance of a 2028 change in control in its 10-Q filing from April.
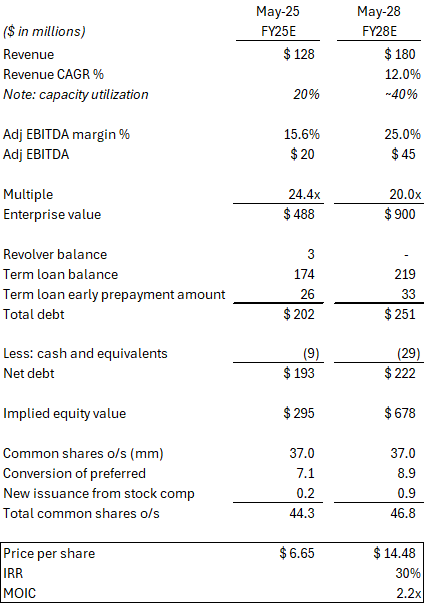
If no near-term transaction materializes, LFCR could still work out to be a good investment. That said, I do have some concerns around valuation – specifically, what multiple LFCR might trade for in three years as it realizes some of the growth embedded in its current valuation and as other players in the industry bring on additional capacity. LFCR currently trades at 24x Adj EBITDA. On the face of it, this does not look cheap, and we are clearly paying something for growth. While I believe this growth is real and the multiple is more than supported by recent M&A activity, I am not sure what premium will still exist in three years in a less supply-constrained environment.
Company overview/background
LFCR is a third-party service provider to pharmaceutical companies, handling the development, manufacturing, and regulatory compliance of pharmaceutical drugs. Within the world of CDMOs, LFCR is focused on the “fill-finish” of injectable products in syringes, vials, and cartridges; additionally, LFCR manufactures sodium hyaluronic (“HA”), which has a broad range of medical uses.
CDMOs generally are attractive, high margin businesses when operating at scale and trade at high multiples due primarily to regulatory constraints. Building out new capacity can take 3-4 years between construction time and regulatory approvals. Once facilities are compliant with the FDA, onboarding and ramping production of new drugs can be an additional 6-24+ month process. “Tech transfers” of commercial drugs are on the shorter end, while development of new drugs has longer lead-times as drugs progress through clinical trials. These regulatory constraints lead to high levels of customer “stickiness”: once a drug has received approvals for production at a specific facility, it is cost and time prohibitive for customers to change suppliers. CDMOs operating at high levels of capacity utilization can have high margins (~30% EBITDA margins) as operating leverage kicks in.
LFCR is currently operating at 20% utilization (and 15% EBITDA margin) after completing a multi-year investment to install a new filler machine, which became GMP-ready and operational in Sep 2024. The filler doubled LFCR’s capacity, and the company estimates its total revenue generating capacity is now $300M.
LFCR recently brought on a new management team with an established track record in the industry. Management took compensation packages that are heavily tied to stock price performance and align them with common stockholders:
Paul Josephs joined as CEO in May 2024, has 30 years of experience in the industry, and has previous experience turning around underperforming CDMOs. Paul stands to make up to ~$1.8M per year in cash comp and time-based stock grants at current share prices, but has potential to make up to an additional $50M in stock grants based on a sliding scale of $7.50-35/share over 5 years.
Ryan Lake joined as CFO in Aug 2024. Ryan previously was CFO for Societal CDMO, a publicly traded CDMO, and oversaw the sale of the company to private equity in Apr 2024. Ryan stands to make ~$1M per year in cash comp and time-based stock grants at current share prices, but has potential to make up to an additional $25M in stock grants based on a sliding scale of $5-30/share over 5 years.
At its investor day in Nov 2024, LFCR laid out a plan to double its capacity utilization to 40% in the next ~2-3 years, which they expect will lead to revenue growing at a 12%+ CAGR and expanding EBITDA margins to 25%+, with minimal new capital investment. A significant portion of this growth is expected to be driven by step-ups in contractual minimum volumes under the terms of supply agreements LFCR has with existing customers. The remainder will come from a pipeline of existing development/tech transfer projects and new business. LFCR currently has 10 active late-stage projects that could obtain FDA approval and commercialization by 2028.
Market/industry background
LFCR operates in fast-growing markets, which should provide a strong demand backdrop for its fill-finish capacity:
The global CDMO market is estimated at $120B and growing at an 8% CAGR.
The global injectable CDMO market (the primary growth engine for LFCR going forward) is estimated at $10B and growing at a 10% CAGR.
The hyaluronic acid market is estimated at $9.8B and growing at a 7% CAGR.
The GLP-1 market (diabetes and weight loss drugs) is estimated at $47B and expected to 10x by 2032.
Fill-finish capacity has been in short supply over the last few years due to the explosion of GLP-1s that are administered via injectables (shots). Pharma companies Novo Nordisk and Eli Lilly, which currently dominate the GLP-1 market, have not been able to keep up with demand. In response to these constraints, both companies have invested historic amounts in capex and acquisitions to secure additional fill-finish capacity:
Novo Holdings acquired Catalent, a publicly traded CDMO, in Dec 2024 for $16.5B (23x Adj EBITDA). As part of the deal, Novo Nordisk simultaneously purchased three of the Catalent fill-finish sites from Novo Holdings for $11B.
Eli Lilly acquired a new injectable manufacturing facility in April 2024 and later, in Dec 2024, announced a $3B expansion of the facility.
Eli Lilly further announced in Feb 2025 plans to double its US manufacturing investment since 2020, to now exceed $50B. The new investment included three new sites for API manufacturing and a fourth injectable site.
LFCR management believes they have the capability to supply GLP-1s, but regardless whether they participate directly or not, GLP-1s have soaked up prefilled syringe capacity and led to a situation where there is more demand than available fill-finish capacity. Interestingly, the Catalent acquisition also could increase churn amongst Catalent’s existing customers who may not want to outsource their supply to a competitor (Novo Nordisk).
Valuation
At first glance, LFCR does not appear cheap trading at 24x Adj EBITDA (trailing). However, recent M&A activity indicates these assets can trade at or above these multiples.
Most recently, Avid Bioservices Inc., a public biologics CDMO, was acquired by GHO Capital & Ampersand Capital in Feb 2025 for 43x Adj EBITDA (trailing). Avid is similar size and has a similar projected financial profile to LFCR. Per a deal presentation, Avid expected to grow revenue from $158M in 2025 to $283M in 2029 (15% CAGR), while expanding EBITDA margin from 15% to 26%. Similar to LFCR, Avid had invested in its manufacturing capacity and estimated it had total revenue generating capacity of $400M. The same 43x multiple applied to LFCR today would put the stock at close to $15/share.
Catalent Inc., a larger publicly traded CDMO, was acquired by Novo Holdings in Dec 2024 for $16.5B or 23x Adj EBITDA (trailing). Notably, Catalent was a much larger and slower growing company relative to LFCR but still achieved a healthy multiple.
Societal CDMO Inc., another publicly traded CDMO, was acquired by CoreRx in Apr 2024 for $160M or 27x Adj EBITDA (trailing). Ryan Lake, the current CFO of LFCR, served as CFO of Societal CDMO through the sale.
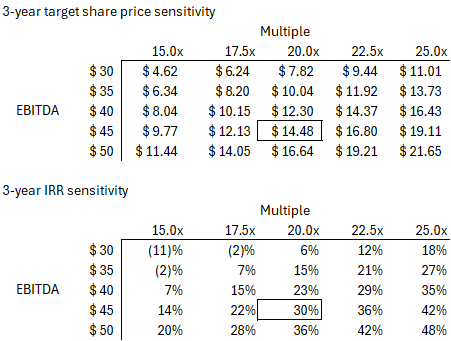
Assuming LFCR management can hit its targets, a 20x multiple would put the stock above $14/share in FY28, or a 30% IRR. See below.
As I mentioned at the outset, the valuation multiple here gives me some pause – how can a 20x multiple or higher ever make sense for a manufacturing business? My view is that a few factors are driving this for both LFCR and the broader industry:
Growth runway from existing capacity – embedded in the current multiple of 24x Adj EBITDA is a runway of growth that should come with very little incremental capital investment. LFCR is operating at 20% utilization and estimates its current capacity supports annual revenue of $300M. At a 25% margin, this equates to EBITDA of $75M or almost 4x current EBITDA.
Optionality to expand capacity and regulatory “know-how” – I believe LFCR’s current facility footprint could support additional capacity. LFCR had previously ordered a 10-head filler in addition to the 5-head filler that became operational in Sep 2024; however, they sold this filler before it was installed in Jan 2025 to raise additional cash. Expanding capacity in the existing facility would be significantly faster and more cost effective than building a new greenfield facility due to both physical construction timing and regulatory reasons. This optionality should be worth something even if it’s not reflected in the current earnings.
Supply constraints – Novo Nordisk and Eli Lilly have not been able to keep up with demand. Both of their GLP-1 products were on the FDA’s drug shortage list since 2022, and one of the key constraints has been fill-finish capacity. These constraints have eased recently (at least for the GLP-1 makers), but in a supply constrained environment where it can take 3-4 years to bring new capacity online, there is going to be a premium placed on available capacity.
Customer stickiness/long-term contracts – high switching costs for customers and long-term supply contracts with annual minimums create consistency in cash flows for CDMOs that should deserve some elevated multiple.
Assuming the company hits their mid-term target (~$45M of EBITDA in FY28), the investment would still work out okay at a slightly lower ~17x multiple. See sensitivity analysis below.
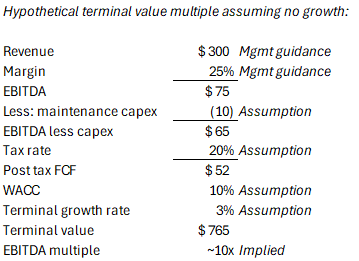
As a sense-check based purely on fundamental cash flow generation, I would estimate a lower bound multiple for a business like LFCR to be something closer to 10x EBITDA assuming it was already operating at full capacity and not assigning value to any future growth. See below. Clearly a higher multiple should apply to LFCR in FY28 given (i) it will still have significant growth runway and (ii) regulatory constraints give it an edge over new entrants, but the current multiple does assign some value to that growth.
Other risks and uncertainties
Business plan execution risk and capital structure – management’s plan looks achievable, but it will be important to monitor the progress of the company’s sales pipeline quarter-to-quarter. In this case, the company’s term loan is currently PIK-ing at 10% and the preferred stock is PIK-ing at 7.5%, so any delay would result in less value accruing to the common shares.
Signs that capacity constraints for fill-finish are easing – Eli Lilly and Novo Nordisk’s GLP-1 drugs were recently taken off FDA’s shortage list in Oct 2024 and Feb 2025. Both companies have invested historic amounts in capex and M&A and have new fill-finish capacity coming online in the next few years. While LFCR does not need to participate directly in GLP-1s to execute its plan, this could have implications for the exit multiple for LFCR.
Oral GLP-1s – both Eli Lilly and Novo Nordisk have oral GLP-1s in development that could receive commercial approval as early as end of this year. All else equal, oral GLP-1s will ease constraints on injectable manufacturing capacity and could result in a lower multiple.
Regulatory environment – there are cross currents here between (i) regulations that encourage domestic pharmaceutical manufacturing and, alternatively (ii) regulations that put increased pressure on pharmaceutical drug pricing (such as the recent executive order calling for a “Most-Favored-Nation” pricing model). Pharmaceutical manufacturing is increasingly being viewed as a strategic industry in the US as indicated by proposed legislation such as the BIOSECURE Act, which likely provides a tailwind for LFCR. Alternatively, increased pressures on drug pricing could result in pharmaceutical companies putting more pressure on CDMOs to lower pricing, which could eat into margins.
Conclusion
While I think LFCR finally has all the pieces in place to execute its business plan, I still struggle a bit with the valuation here despite recent industry comps. I do think there is a decent chance that a buyer emerges for LFCR in the near-term which would likely result in a good outcome from current prices. Longer-term I’m not as confident in underwriting an exit multiple, but the investment would still work out okay with some multiple contraction. Given the uncertainty, I have sized this position a bit smaller.
Disclosure: I own shares of LFCR